Sacral Neuromodulation
The urinary bladder has two functions: The first is to store urine coming from the kidneys in a comfortable manner. The second function is to void urine voluntarily and completely. A very important anatomical structure is the external urinary sphincter that needs a coordination with the muscles of the bladder in order to have a completely functional unit. This coordination and voluntary control of urine is due to a network of central nervous system. When this coordination becomes imbalanced, certain signs and symptoms show up that need to be treated. Sacral neuromodulation is used in France and United States for this type of urologic indications since few years. This latest treatment is used for refractory, chronic conditions in patients with overactive bladder and patients with urinary retention (non-obstructive). It is recently used in non-urologic cases as fecal incontinence and constipation. It is considered the last resort for urgency / urge incontinence after failure/intolerance of medical treatment and/or injection of botulinum toxin A in the bladder. Besides, it is used for urinary retention (majorly in women) with or without sphincter anomalies. This technique showed great results in young females with unexplained urinary retention with non-relaxation of the sphincter.
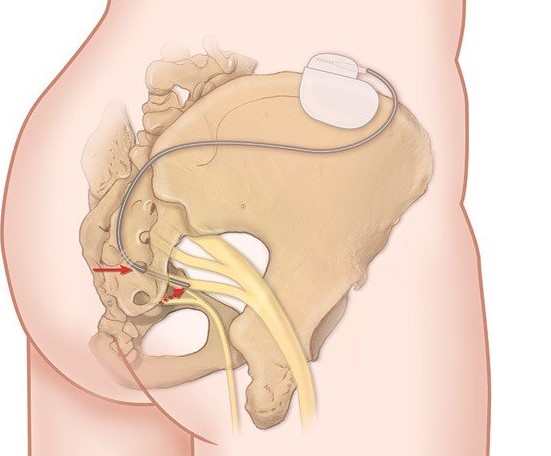

This procedure is done when the urologist inserts an electrode in the S3 sacral foramen (from the lower back) and connects the electrode to an external stimulator. This electrode transmits signals (current) to S3 which in turn modifies the signals transmitted by this nervous network (reset of the system) for better control of the bladder and sphincter. This technique is very conservative in the meaning that nothing is destroyed or removed, and it is reversible as the effects stop when the stimulator is stopped. A testing phase is usually done for 2-3 weeks and if the patient reports >50% improvement in symptoms; a definitive battery (stimulator) is placed in the lower back with a very small incision. The testing phase takes about 60 minutes (operating time) and the definitive phase of the procedure about 30 minutes.
More Specializations
Laparoscopic surgery allows the surgeon to perform complex surgeries through small incisions. Recovery is very quick and with minimal pain as tiny incisions are being made. Very minimal scarring and quite esthetic.
Read more
Laser surgery for the prostate is a revolution that allows us to perform surgeries to the prostate while the patient is on blood thinners without the need to stop them, especially in patients who need to be continuously on such medications like Aspirin etc.
Read more
Laser surgery for kidney stones eradicates the need to perform painful incisions in order to remove kidney stones and gives the advantage of being very accurate and incision-free in most cases.
Read more
Sexual dysfunction in men.
Read more
Excess weight is associated with many comorbid conditions, such as heart disease, cholesterol imbalances, diabetes, and the Metabolic Syndrome.
Read more
Worldwide, diabetes is on the rise, majorly due to an unhealthy lifestyle as well as predisposing factors.
Read more
More Specializations
Laparoscopic surgery allows the surgeon to perform complex surgeries through small incisions. Recovery is very quick and with minimal pain as tiny incisions are being made. Very minimal scarring and quite esthetic.
Read more
Laser surgery for the prostate is a revolution that allows us to perform surgeries to the prostate while the patient is on blood thinners without the need to stop them, especially in patients who need to be continuously on such medications like Aspirin etc.
Read more
Laser surgery for kidney stones eradicates the need to perform painful incisions in order to remove kidney stones and gives the advantage of being very accurate and incision-free in most cases.
Read more
Sexual dysfunction in men.
Read more
Excess weight is associated with many comorbid conditions, such as heart disease, cholesterol imbalances, diabetes, and the Metabolic Syndrome.
Read more
Worldwide, diabetes is on the rise, majorly due to an unhealthy lifestyle as well as predisposing factors.
Read more
